INDICATION:
What is TUKYSA? TUKYSA is a prescription medicine used to treat adults with:
- a type of breast cancer called human epidermal growth factor receptor-2 (HER2) positive breast cancer. TUKYSA is used with the medicines trastuzumab and capecitabine, when your cancer has spread to other parts of the body such as the brain (metastatic), or cannot be removed by surgery, and you have received one or more anti-HER2 breast cancer treatments.
- a type of colorectal cancer called RAS wild-type HER2 positive colorectal cancer. TUKYSA is used with the medicine trastuzumab, when your cancer has spread to other parts of the body (metastatic), or cannot be removed by surgery, and you have received treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and it did not work or is no longer working. This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use.
It is not known if TUKYSA is safe and effective in children.
What is TUKYSA? TUKYSA is a prescription medicine used with the medicines trastuzumab and capecitabine to treat adults with human epidermal growth factor receptor-2 (HER2) positive breast cancer that has spread to other parts of the body such as the brain (metastatic), or that cannot be removed by surgery, and who have received one or more anti-HER2 breast cancer treatments. It is not known if TUKYSA is safe and effective in children.
What is TUKYSA? TUKYSA is a prescription medicine used with the medicine trastuzumab to treat adults with RAS wild-type human epidermal growth factor receptor-2 (HER2) positive colorectal cancer that has spread to other parts of the body (metastatic), or cannot be removed by surgery, and who have received treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and it did not work or is no longer working. This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use. It is not known if TUKYSA is safe and effective in children.
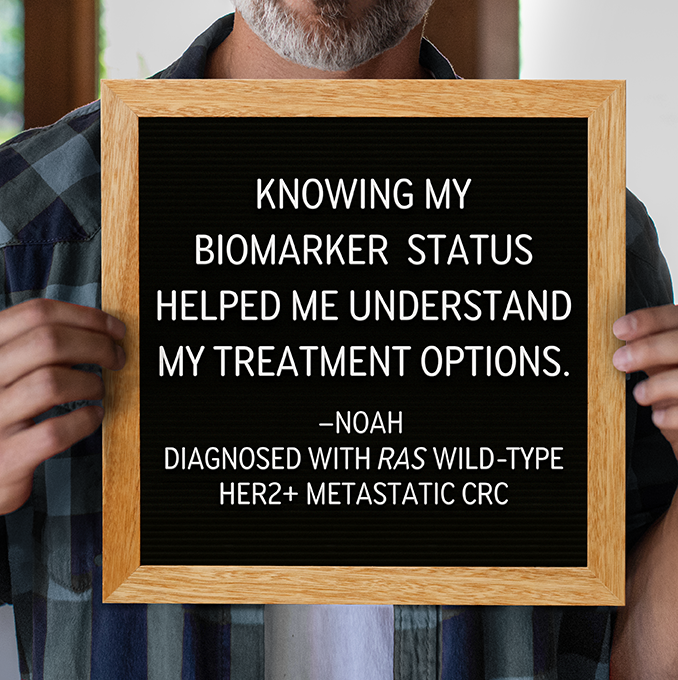
RAS Wild-type, HER2+ Metastatic Colorectal Cancer and the TUKYSA Treatment Regimen
Learning about RAS wild-type, HER2 positive (HER2+) metastatic (also known as stage 4) colorectal cancer and its treatment can be a lot to take in. Here is some information to help you get started.
On this page, you can learn more about:
- Why it's important to know your biomarker status
- HER2 and RAS, biomarkers for metastatic colorectal cancer
- How TUKYSA works with trastuzumab to target HER2
To find out if TUKYSA may be a treatment option for you, talk to your healthcare provider about biomarker testing.
Why Does Knowing My Biomarker Status Matter?
Your HER2 and RAS status can help your healthcare provider decide what treatments may be right for you.
TUKYSA, along with trastuzumab, offers a chance to treat your RAS wild-type, HER2+ metastatic colorectal cancer
HER2 and RAS Are Important Biomarkers for Metastatic Colorectal Cancer
What are biomarkers?
There are different types of colorectal cancer. Each type has different biomarkers. Some biomarkers can be proteins found in cancer cells. They are identified by testing tumor tissue.
Proteins are substances that help the cells in the body work.
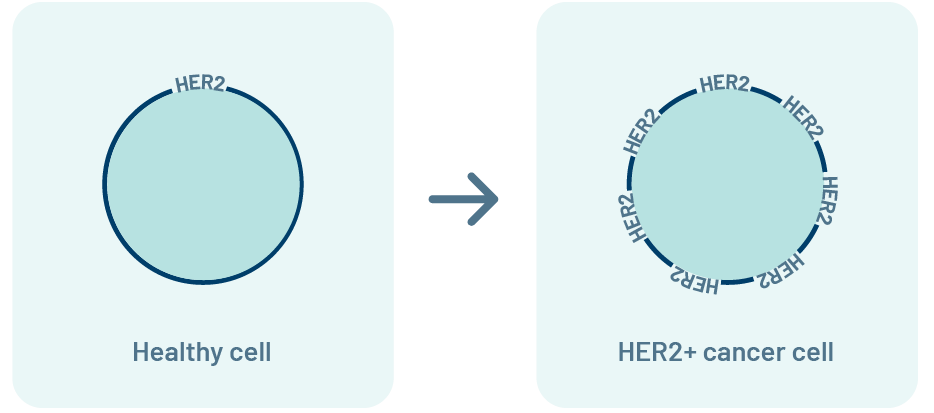
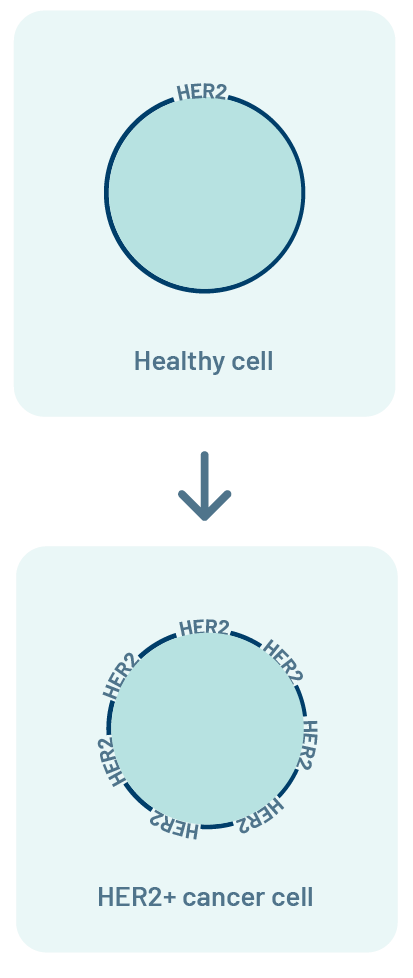
What is the HER2 biomarker?
HER2 is a protein. In healthy cells, HER2 tells cells to grow and
multiply at a normal rate.
Some cancer cells have too many HER2 proteins (HER2+). Having a lot of
HER2 can tell cancer cells to grow and multiply quickly.
What is the RAS biomarker?
Like HER2, RAS is a biomarker.
Sometimes RAS has a defect (also called a mutation) and sometimes it does not. When RAS does not have a defect, it is called RAS wild type (RAS WT).
To be eligible for TUKYSA, you must have tumors that are both RAS wild type and HER2+.
The First FDA-Approved Chemotherapy-Free* Regimen in RAS Wild-type, HER2+ Unresectable or Metastatic Colorectal Cancer
TUKYSA is an oral treatment designed to target HER2. It is taken with another medicine called trastuzumab (also called Herceptin®).
TUKYSA is for adults with RAS wild-type, HER2 positive colorectal cancer that has spread to other parts of the body (metastatic), or cannot be removed by surgery, and who have received treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and it did not work or is no longer working.
It was first approved in 2020 for a different patient population. Visit TUKYSA.com to learn more.
*Although they are not chemotherapy, HER2-targeted therapies can affect normal cells and cause side effects, some of which may be serious.
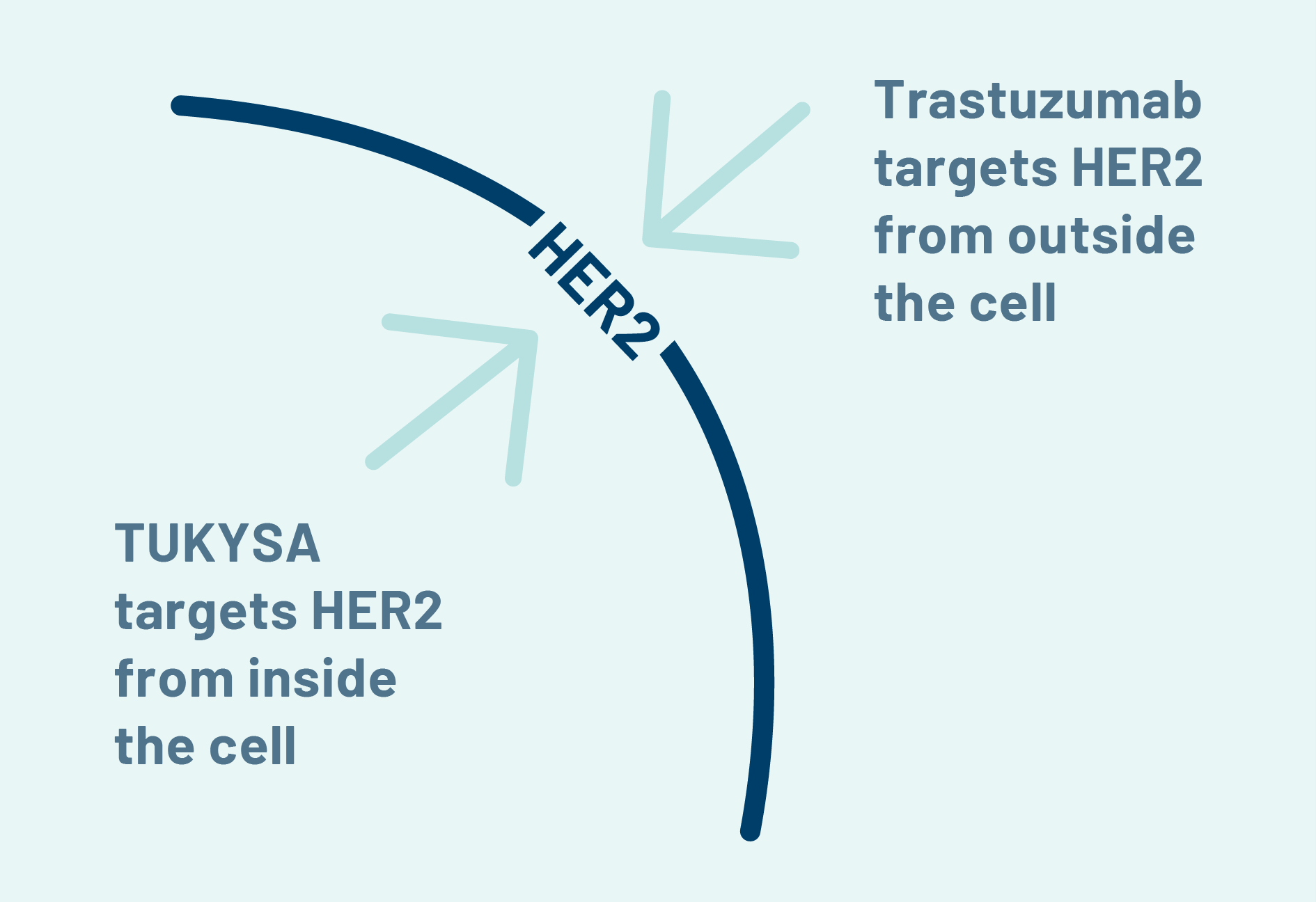
Target HER2 From Inside and Out
TUKYSA and trastuzumab are not chemotherapy.
Both medicines are designed to target HER2, but they do it in different ways. Together, they may work to more completely block the signal that tells cancer cells to grow.
HER2 is also found on normal cells. This means HER2-targeted therapies such as TUKYSA can affect normal cells and cause side effects, some of which may be serious.
Important Safety Information

What are the possible side effects of TUKYSA?
TUKYSA may cause serious side effects, including:
- Diarrhea (watery, loose, or frequent stools) is common and can sometimes be severe. Tell your healthcare provider if you have a change in your bowel movements or severe diarrhea. Severe diarrhea can cause a loss of too much body fluids (dehydration), low blood pressure, kidney problems, and death. Your healthcare provider may prescribe medicines to treat your diarrhea during treatment with TUKYSA.
- Liver Problems, including severe cases. Your healthcare provider will test your blood to check your liver function before starting and every 3 weeks during treatment with TUKYSA, or as needed. Tell your healthcare provider right away if you have any signs and symptoms of liver problems including itching, yellowing of your skin or eyes, dark or brown urine (tea-colored), pain in the right upper stomach area (abdomen), feeling very tired, decreased appetite, or bleeding or bruising more easily than normal.
The most common side effects of TUKYSA in combination with trastuzumab and capecitabine in adults with HER2-positive breast cancer include:
- diarrhea
- rash, redness, pain, swelling, or blisters on the palms of your hands or soles of your feet
- nausea
- increased liver function blood tests
- vomiting
- mouth sores (stomatitis)
- decreased appetite
- a low number of red blood cells (anemia)
- rash
The most common side effects of TUKYSA in combination with trastuzumab in adults with RAS wild-type HER2-positive colorectal cancer include:
- diarrhea
- tiredness
- rash
- nausea
- stomach-area (abdomen) pain
- infusion-related reactions
- fever
TUKYSA may cause fertility problems in males and females, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of TUKYSA. Discuss side effects with your healthcare provider. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/Safety/MedWatch.
These are not all the possible side effects of TUKYSA. Discuss side effects with your healthcare provider. You may report negative side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/Safety/MedWatch.

What should I tell my healthcare provider before taking TUKYSA?
Before taking TUKYSA, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
-
are pregnant or plan to become pregnant. TUKYSA can harm your unborn
baby.
Females who can become pregnant: Your healthcare provider will do a pregnancy test before you start taking TUKYSA. Use effective birth control (contraception) during TUKYSA treatment and for 1 week after the last dose of TUKYSA. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with TUKYSA.
Males with a female partner who can become pregnant: Use effective birth control during TUKYSA treatment and for 1 week after the last dose of TUKYSA. - are breastfeeding (nursing) or plan to breastfeed. Do not breastfeed during treatment with TUKYSA and for 1 week after the last dose of TUKYSA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. TUKYSA may affect the way your other medicines work, and other medicines may affect the way TUKYSA works. Keep a list of all the medicines you take and show it to your healthcare provider and pharmacist every time you get a new medicine.
REF-8290_FINAL_01/23
Indication
Indication

What is TUKYSA?
TUKYSA is a prescription medicine used with the medicine trastuzumab to treat adults with RAS wild-type human epidermal growth factor receptor-2 (HER2) positive colorectal cancer that has spread to other parts of the body (metastatic), or cannot be removed by surgery, and who have received treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and it did not work or is no longer working.
This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use.
It is not known if TUKYSA is safe and effective in children.
Please see Important Facts about TUKYSA.
TUKYSA is a prescription medicine used to treat adults with:
- a type of breast cancer called human epidermal growth factor receptor-2 (HER2) positive breast cancer. TUKYSA is used with the medicines trastuzumab and capecitabine, when your cancer has spread to other parts of the body such as the brain (metastatic), or cannot be removed by surgery, and you have received one or more anti-HER2 breast cancer treatments.
-
a type of colorectal cancer called RAS wild-type HER2
positive colorectal cancer. TUKYSA is used with the medicine
trastuzumab, when your cancer has spread to other parts of the
body (metastatic), or cannot be removed by surgery,
and you have received treatment with
fluoropyrimidine-, oxaliplatin-, and irinotecan-based
chemotherapy and it did not work or is no longer working.
This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use.
It is not known if TUKYSA is safe and effective in children.
Please see Important Facts about TUKYSA.
TUKYSA is a prescription medicine used with the medicines trastuzumab and capecitabine to treat adults with human epidermal growth factor receptor-2 (HER2) positive breast cancer that has spread to other parts of the body such as the brain (metastatic), or that cannot be removed by surgery, and who have received one or more anti-HER2 breast cancer treatments.
It is not known if TUKYSA is safe and effective in children.
Please see Important Facts about TUKYSA.
Important Safety Information
Important Safety Information and Indication
What are the possible side effects of TUKYSA?
TUKYSA may cause serious side effects, including:
- Diarrhea (watery, loose, or frequent stools) is common and can sometimes be severe. Tell your healthcare provider if you have a change in your bowel movements or severe diarrhea. Severe diarrhea can cause a loss of too much body fluids (dehydration), low blood pressure, kidney problems, and death. Your healthcare provider may prescribe medicines to treat your diarrhea during treatment with TUKYSA.
- Liver Problems, including severe cases. Your healthcare provider will test your blood to check your liver function before starting and every 3 weeks during treatment with TUKYSA, or as needed. Tell your healthcare provider right away if you have any signs and symptoms of liver problems including itching, yellowing of the skin or eyes, dark or brown urine (tea-colored), pain or discomfort in the right upper stomach area (abdomen), feeling very tired, decreased appetite, or bleeding or bruising more easily than normal.
What is TUKYSA?
TUKYSA is a prescription medicine used to treat adults with:
- a type of breast cancer called human epidermal growth factor receptor-2 (HER2) positive breast cancer. TUKYSA is used with the medicines trastuzumab and capecitabine, when your cancer has spread to other parts of the body such as the brain (metastatic), or cannot be removed by surgery, and you have received one or more anti-HER2 breast cancer treatments.
-
a type of colorectal cancer called RAS wild-type HER2
positive colorectal cancer. TUKYSA is used with the medicine
trastuzumab, when your cancer has spread to other parts of the
body (metastatic), or cannot be removed by surgery,
and you have received treatment with
fluoropyrimidine-, oxaliplatin-, and irinotecan-based
chemotherapy and it did not work or is no longer working.
This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use.
It is not known if TUKYSA is safe and effective in children.
What is TUKYSA? TUKYSA is a prescription medicine used with the medicines trastuzumab and capecitabine to treat adults with human epidermal growth factor receptor-2 (HER2) positive breast cancer that has spread to other parts of the body such as the brain (metastatic), or that cannot be removed by surgery, and who have received one or more anti-HER2 breast cancer treatments. It is not known if TUKYSA is safe and effective in children.
What is TUKYSA? TUKYSA is a prescription medicine used with the medicine trastuzumab to treat adults with RAS wild-type human epidermal growth factor receptor-2 (HER2) positive colorectal cancer that has spread to other parts of the body (metastatic), or cannot be removed by surgery, and who have received treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and it did not work or is no longer working. This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use. It is not known if TUKYSA is safe and effective in children.

What are the possible side effects of TUKYSA?
TUKYSA may cause serious side effects, including:
- Diarrhea (watery, loose, or frequent stools) is common and can sometimes be severe. Tell your healthcare provider if you have a change in your bowel movements or severe diarrhea. Severe diarrhea can cause a loss of too much body fluids (dehydration), low blood pressure, kidney problems, and death. Your healthcare provider may prescribe medicines to treat your diarrhea during treatment with TUKYSA.
- Liver Problems, including severe cases. Your healthcare provider will test your blood to check your liver function before starting and every 3 weeks during treatment with TUKYSA, or as needed. Tell your healthcare provider right away if you have any signs and symptoms of liver problems including itching, yellowing of your skin or eyes, dark or brown urine (tea-colored), pain in the right upper stomach area (abdomen), feeling very tired, decreased appetite, or bleeding or bruising more easily than normal.
The most common side effects of TUKYSA in combination with trastuzumab and capecitabine in adults with HER2-positive breast cancer include:
- diarrhea
- rash, redness, pain, swelling, or blisters on the palms of your hands or soles of your feet
- nausea
- increased liver function blood tests
- vomiting
- mouth sores (stomatitis)
- decreased appetite
- a low number of red blood cells (anemia)
- rash
The most common side effects of TUKYSA in combination with trastuzumab in adults with RAS wild-type HER2-positive colorectal cancer include:
- diarrhea
- tiredness
- rash
- nausea
- stomach-area (abdomen) pain
- infusion-related reactions
- fever
Your healthcare provider may change your dose of TUKYSA, temporarily stop, or permanently stop treatment with TUKYSA if you have certain side effects.
TUKYSA may cause fertility problems in males and females, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of TUKYSA. Discuss side effects with your healthcare provider. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/Safety/MedWatch.
These are not all the possible side effects of TUKYSA. Discuss side effects with your healthcare provider. You may report negative side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/Safety/MedWatch.

What should I tell my healthcare provider before taking TUKYSA?
Before taking TUKYSA, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
-
are pregnant or plan to become pregnant. TUKYSA can harm your
unborn baby.
Females who can become pregnant: Your healthcare provider will do a pregnancy test before you start taking TUKYSA. Use effective birth control (contraception) during TUKYSA treatment and for 1 week after the last dose of TUKYSA. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with TUKYSA.
Males with a female partner who can become pregnant:
Males with a female partner who can get pregnant: Use effective birth control during TUKYSA treatment and for 1 week after the last dose of TUKYSA. - are breastfeeding (nursing) or plan to breastfeed. Do not breastfeed during treatment with TUKYSA and for 1 week after the last dose of TUKYSA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. TUKYSA may affect the way your other medicines work, and other medicines may affect the way TUKYSA works. Keep a list of all the medicines you take and show it to your healthcare provider and pharmacist every time you get a new medicine.
REF-8273_FINAL_01/23

Indication
What is TUKYSA?
TUKYSA is a prescription medicine used to treat adults with:
- a type of breast cancer called human epidermal growth factor receptor-2 (HER2) positive breast cancer. TUKYSA is used with the medicines trastuzumab and capecitabine, when your cancer has spread to other parts of the body such as the brain (metastatic), or cannot be removed by surgery, and you have received one or more anti-HER2 breast cancer treatments.
-
a type of colorectal cancer called RAS wild-type HER2
positive colorectal cancer. TUKYSA is used with the medicine
trastuzumab, when your cancer has spread to other parts of
the body (metastatic), or cannot be removed by surgery,
and you have received treatment with
fluoropyrimidine-, oxaliplatin-, and irinotecan-based
chemotherapy and it did not work or is no longer working.
This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use.
It is not known if TUKYSA is safe and effective in children.
Please see Important Facts about TUKYSA.

What is TUKYSA?
TUKYSA is a prescription medicine used with the medicines trastuzumab and capecitabine to treat adults with human epidermal growth factor receptor-2 (HER2) positive breast cancer that has spread to other parts of the body such as the brain (metastatic), or that cannot be removed by surgery, and who have received one or more anti-HER2 breast cancer treatments.
It is not known if TUKYSA is safe and effective in children.
Please see Important Facts about TUKYSA.

What is TUKYSA?
TUKYSA is a prescription medicine used with the medicine trastuzumab to treat adults with RAS wild-type human epidermal growth factor receptor-2 (HER2) positive colorectal cancer that has spread to other parts of the body (metastatic), or cannot be removed by surgery, and who have received treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and it did not work or is no longer working.
This use is approved based on a clinical study that measured how many patients had a tumor response and how long that response lasted. Studies are ongoing to confirm the benefit of TUKYSA for this use.
It is not known if TUKYSA is safe and effective in children.
Please see Important Facts about TUKYSA.
